Methods
Between 2022–2024, 344 intent-to-treat (ITT) patients were enrolled (320 treated per-protocol (PP)) at 53 US sites. The primary endpoint for this single-arm, post-approval study is a composite of major adverse events (stroke, death, or myocardial infarction (MI)) through 30 days post-procedure, plus ipsilateral stroke from day 31 to 365 post-procedure. The incidence of cranial nerve injury (CNI) within 30 days post-procedure is a key secondary endpoint. Independent neurological assessments are performed for all patients before the procedure, within 24 hours, at 30-days, and at 1-year after TCAR. Events were adjudicated by an independent clinical events committee.
Findings
In the ITT population, 75.3% were less than 75 years of age, 42.7% were female, and 16.3% were symptomatic. Among symptomatic patients, 25.0% experienced a neurologic event within 2 weeks preceding the TCAR procedure. The mean lesion length was 23.3mm, 47.4% had a Type II or Type III aortic arch, and 17.2% of lesions had severe calcification.
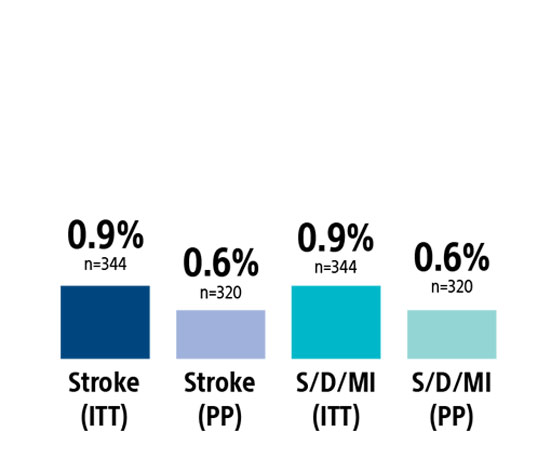
In the ITT population, the rate of stroke/death/MI at 30-days was 0.9% (0.6% PP) with a 30-day stroke rate of 0.9% (0.6% PP, n=2). There were no deaths or MIs through 30-day follow-up. The incidence of CNI within 30 days was 0.6% (0.6% PP); both resolved within 6 months.
Conclusions
These 30-day results of the ROADSTER 3 study demonstrate that TCAR, using the ENROUTE TSS in conjunction with the ENROUTE NPS, is safe and effective in patients at standard risk for adverse events from carotid endarterectomy.